- This element is ____.

- silver
- aluminum
- gold
- arsenic
- This element is ____.

- argon
- silver
- astatine
- aluminum
- This element is ____.

- arsenic
- argon
- actinium
- americium
- This element is ____.

- arsenic
- silver
- argon
- aluminum
- This element is ____.

- actinium
- uranium
- silver
- gold
- This element is ____.
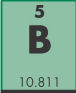
- beryllium
- bismuth
- bohrium
- boron
- This element is ____.

- barium
- bismuth
- boron
- lead
- This element is ____.

- barium
- boron
- beryllium
- bismuth
- This element is ____.

- bismuth
- beryllium
- bromine
- barium
- This element is ____.

- bromine
- beryllium
- bohrium
- barium
- This element is ____.

- calcium
- carbon
- cobalt
- copper
- This element is ____.

- carbon
- calcium
- californium
- cadmium
- This element is ____.

- cadmium
- chlorine
- calcium
- carbon
- This element is ____.

- chlorine
- californium
- curium
- cobalt
- This element is ____.

- chlorine
- carbon
- copper
- cobalt
- This element is ____.

- chromium
- carbon
- cerium
- chlorine
- This element is ____.

- cerium
- cadmium
- cesium
- chromium
- This element is ____.

- cerium
- cesium
- carbon
- copper
- This element is ____.

- einsteinium
- erbium
- europium
- helium
- This element is ____.

- fluorine
- iron
- francium
- palladium
- This element is ____.

- fluorine
- iron
- fermium
- iodine
- This element is ____.

- cobalt
- gadolinium
- germanium
- gallium
- This element is ____.

- gallium
- gadolinium
- germanium
- gold
- This element is ____.

- hydrogen
- halmium
- helium
- hafnium
- This element is ____.

- hafnium
- hassium
- hydrogen
- helium
- This element is ____.

- mercury
- hafnium
- hydrogen
- holmium
- This element is ____.

- iron
- iridium
- iodine
- zinc
- This element is ____.

- krypton
- potassium
- berkelium
- xenon
- This element is ____.

- krypton
- potassium
- chromium
- argon
- This element is ____.

- lithium
- iodine
- lutelium
- lawrencium
- This element is ____.

- magnesium
- manganese
- molybdenum
- meitnerium
- This element is ____.

- mendelevium
- mercury
- magnesium
- manganese
- This element is ____.

- nitrogen
- neon
- niobium
- sodium
- This element is ____.

- sodium
- nitrogen
- neon
- nobelium
- This element is ____.

- sodium
- neptunium
- neon
- nickel
- This element is ____.

- nickel
- neon
- neptunium
- nitrogen
- This element is ____.

- oxygen
- osmium
- copper
- sodium
- This element is ____.

- potassium
- phosphorus
- lead
- palladium
- This element is ____.

- polonium
- lead
- phosphorus
- plutonium
- This element is ____.

- rutherfordium
- rubidium
- rhenium
- radon
- This element is ____.

- sulfur
- strontium
- antimony
- silicon
- This element is ____.
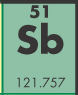
- antimony
- tin
- seabargium
- selenium
- This element is ____.

- strontium
- tin
- scandium
- sulfur
- This element is ____.

- silicon
- silver
- strontium
- selenium
- This element is ____.

- selenium
- silver
- tin
- silicon
- This element is ____.

- tin
- strontium
- selenium
- silicon
- This element is ____.

- silver
- sulfur
- scandium
- strontium
- This element is ____.

- tin
- thorium
- titanium
- terbium
- This element is ____.

- uranium
- plutonium
- neptunium
- mercury
- This element is ____.

- uranium
- vanadium
- lutetium
- meitnerium
- This element is ____.

- xenon
- xerox
- The chemical symbol for this is _____.

- H
- He
- Ni
- Hy
- The chemical symbol for this is _____.

- He
- H
- Hl
- Hm
- The chemical symbol for this is _____.

- Lt
- Li
- L
- Lm
- The chemical symbol for this is _____.

- Br
- Bi
- Bl
- Be
- The chemical symbol for this is _____.

- By
- Br
- Bo
- B
- The chemical symbol for this is _____.

- C
- Ca
- Cb
- Cr
- The chemical symbol for this is _____.

- Ne
- Nt
- Ni
- N
- The chemical symbol for this is _____.

- Oz
- On
- Ox
- O
- The chemical symbol for this is _____.

- F
- Fl
- Fn
- Fe
- The chemical symbol for this is _____.
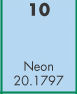
- N
- Ni
- Ne
- Xe
- The chemical symbol for this is _____.

- Ni
- Sd
- Na
- So
- The chemical symbol for this is _____.

- Mg
- Mn
- Ma
- Ms
- The chemical symbol for this is _____.

- Tn
- Al
- Sn
- Am
- The chemical symbol for this is _____.

- Si
- Sl
- S
- Sn
- The chemical symbol for this is _____.

- Po
- Ps
- Ph
- P
- The chemical symbol for this is _____.

- Sl
- Sf
- Su
- S
- The chemical symbol for this is _____.

- Cl
- Ch
- C
- Ci
- The chemical symbol for this is _____.

- An
- A
- Ag
- Ar
- The chemical symbol for this is _____.

- P
- Pt
- Po
- K
- The chemical symbol for this is _____.

- Ca
- Cl
- C
- Cm
- The chemical symbol for this is _____.

- Sa
- Sc
- Sd
- Sn
- The chemical symbol for this is _____.

- Tt
- Tn
- T
- Ti
- The chemical symbol for this is _____.

- Vn
- Vd
- Va
- V
- The chemical symbol for this is _____.

- Co
- Cm
- Ch
- Cr
- The chemical symbol for this is _____.

- Mn
- Mg
- M
- Ma
- The chemical symbol for this is _____.

- Ir
- Fe
- Io
- I
- The chemical symbol for this is _____.

- Co
- C
- Cb
- Ca
- The chemical symbol for this is _____.

- Fe
- Co
- Cu
- Sn
- The chemical symbol for this is _____.

- Ga
- Gl
- G
- Gi
- The chemical symbol for this is _____.

- As
- Ar
- An
- Ac
- The chemical symbol for this is _____.

- B
- Br
- Bm
- Bo
- The chemical symbol for this is _____.

- Sr
- St
- So
- Sn
- The chemical symbol for this is _____.

- Ti
- Tn
- Pb
- Sn
- The chemical symbol for this is _____.

- Io
- Id
- I
- In
- The chemical symbol for this is _____.

- Cs
- Ce
- Cm
- Ci
- The chemical symbol for this is _____.

- Au
- Gd
- Gl
- Ad
- The chemical symbol for this is _____.

- Pb
- Ld
- Le
- Fe
- The chemical symbol for this is _____.

- U
- Ur
- Un
- Um
- The chemical symbol for this is _____.

- Nk
- N
- Nl
- Ni
- The chemical symbol for this is _____.

- An
- Zn
- Zc
- Zi
- The chemical symbol for this is _____.

- Ge
- Gr
- Gm
- Gn
- The chemical symbol for this is _____.

- Se
- Sl
- Sn
- Sm
- The chemical symbol for this is _____.

- Kr
- Kp
- Kt
- Kn
- The chemical symbol for this is _____.

- Ag
- Si
- Sr
- Au
- The chemical symbol for this is _____.

- Sb
- An
- At
- Sn
- The chemical symbol for this is _____.

- Xe
- Xn
- Xo
- X
- The chemical symbol for this is _____.

- Br
- Ba
- Bm
- Bi
- The chemical symbol for this is _____.

- Hg
- Hm
- Me
- Mc
- The chemical symbol for this is _____.

- Rd
- Ra
- Ri
- Rn
- The chemical symbol for this is _____.

- Pn
- Pt
- Pl
- Pu
- The red arrows in the figure below point to numbers. These numbers indicate _____.
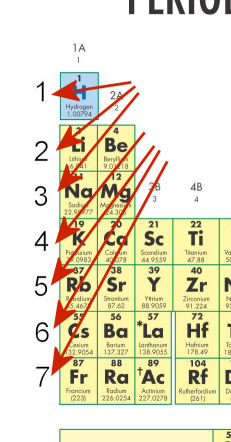
- periods
- atomic mass
- families
- atomic number
- The red arrows in the figure below point to numbers. These numbers indicate _____.
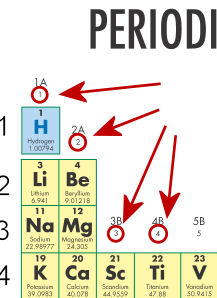
- families
- atomic mass
- periods
- atomic number
- The red arrow in this diagram is pointing to a number that indicates the _____.

- atomic number
- atomic mass
- chemical symbol
- family
- The red arrow in this diagram is pointing to a number that indicates the _____.

- atomic number
- atomic mass
- period
- family
- The circled element in the figure below is shown in a different font color than surrounding elements. Why is it a different color?
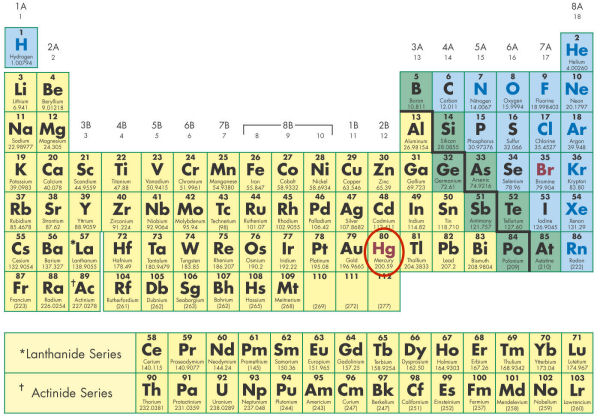
- It is the only metal which is in a liquid state at room temperature.
- It is the only metal in a solid state at room temperature.
- It is the only liquid non-metal in a liquid state at room temperature.
- It is the only non-metal in a solid state at room temperature.
- The circled element in the figure below is shown in a different font color than surrounding elements. Why is it a different color?
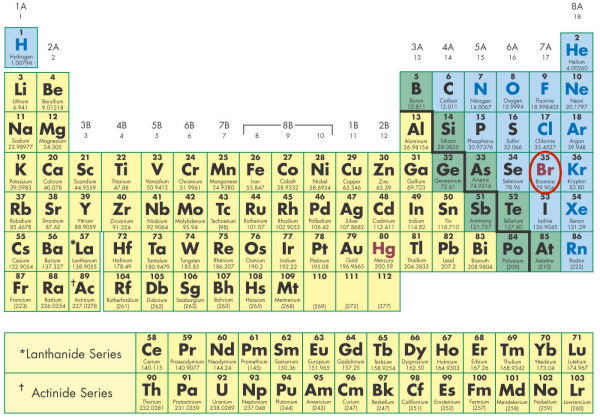
- It is the only metal which is in a liquid state at room temperature.
- It is the only metal in a solid state at room temperature.
- It is the only liquid non-metal in a liquid state at room temperature.
- It is the only non-metal in a solid state at room temperature.
- There is a gap in the area circled in the diagram below. Why is that gap there?
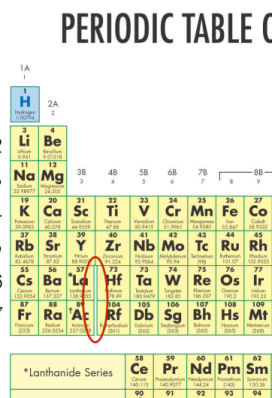
- The four elements in that area behave mysteriously. This gap indicates a gap in scientific knowledge related to those elements to-date.
- There is a gap in atomic masses between these neighboring elements.
- The elements that belong in this area are still a scientific mystery.
- This is where the two rows at the bottom of the Periodic Table would belong. They are moved to the bottom of the table to save space.
- The bold black line pointed out in the diagram below is there for a reason. What is that reason?
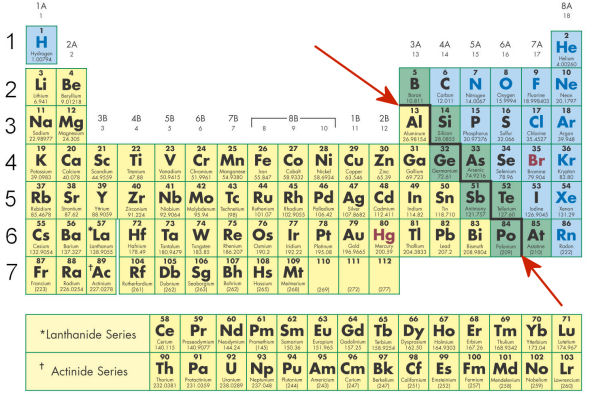
- This line separates liquids and gases.
- This line separates metals and non-metals.
- This line separates solids and liquids.
- This line separates metals and gases.
- The most active metal is _____.
-
- The most active non-metal is _____.
-
- Hydrogen, by its electron arrangement, is part of Group 1. It is set apart on the Periodic Table because _____.
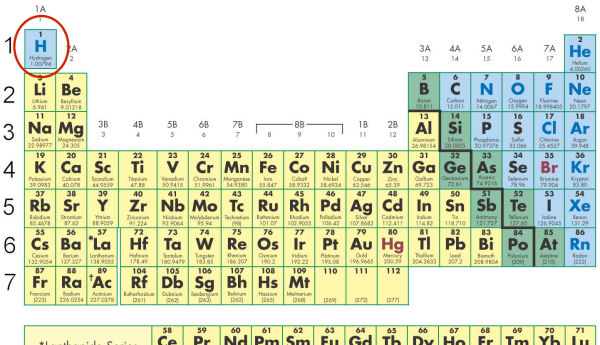
- its state is everchanging, and it does not quite fit the attached to the remaining elements
- it was put in as an afterthought
- it has only one energy level and has unique properties
- it is the most important element
- The elements circled below are shaded green, while the elements to the left are yellow, and to the right are blue. Why are these elements shaded green in color?
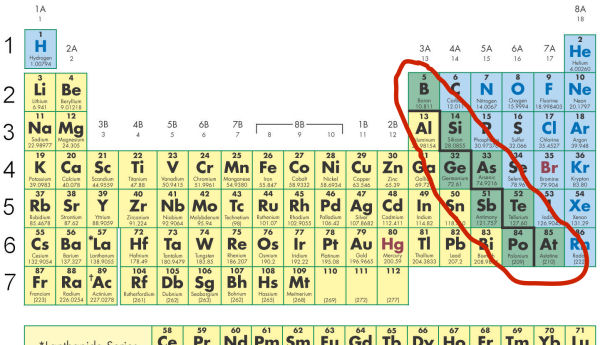
- They are metalloid, containing some, but not all of the properties of metals.
- The designer of the Periodic Table thought it would make the diagram more interesting.
- These elements are precious.
- Their properties are known to be unstable.
- The red arrow in the diagram below is pointing to the _____.
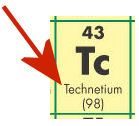
- element's name
- chemical symbol
- atomic mass
- atomic number
- The chemical symbol for this is _____.

- En
- Es
- Ei
- Mj



