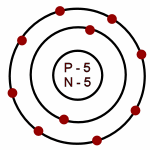
The smallest piece of matter was named "atom" by _____.
- Socrates
- Nero
- Democritus
- Hippocrates
The electron was discovered by _____.
- John Dalton
- J.J. Thomson
- Ernest Rutherford
- Niels Bohr
Bohr's atomic model placed the electrons _____.
- in the nucleus
- in definite orbits around the nucleus
- in no definite position
- scattered among the positive charges
Almost all the mass of an atom is found _____.
- outside the nucleus
- in the electron cloud
- in the energy levels
- in the nucleus
The nucleus of an atom contains _____.
- protons and electrons
- protons and neutrons
- electrons and neutrons
- only neutrons
The atomic number of an element indicates the _____.
- sum of protons plus neutrons
- sum of protons plus electrons
- number of protons
- number of neutrons
The atomic number of magnesium is 12. Its nucleus must contain _____.
- 6 protons and 6 neutrons
- 6 neutrons and 6 electrons
- 12 protons and no electrons
- 12 neutrons and no protons
The atomic number of hydrogen is 1. Its nucleus must contain _____.
- 1 proton and 1 electron
- 1 proton only
- 1 electron only
- 1 neutron only
An element has an atomic number of 4 and atomic weight (mass) of 9. The number of neutrons in the nucleus of this atom is _____.
- 2
- 3
- 4
- 5
The word atom comes from the Greek word atomos, meaning _____.
- matter
- indivisible
- mass
- volume
All neutrons _____.
- are electrically neutral
- have a mass of 2 amu
- are electrically positive
- are located outside the nucleus
Indirect evidence about an object is evidence gathered _____.
- by reading a textbook
- without actually seeing or touching the object
- without any experimenting
- that cannot be used in a theory
The central portion of an atom is called the _____.
- nucleus
- core
- element
- atomsphere
The mass (or weight) of subatomic particles is measured by a special unit called the _____.
- aclu
- pii
- ion
- amu
The Greek word atomos means _____.
- mass
- atmosphere
- invisible
- cannot be divided
The number of protons in an element, also called it _____, identifies the element.
- charge
- atomic number
- nucleus
- mass
The subatomic particle that has a neutral charge is the _____.
- electron
- neutron
- proton
- neuron
In an atom, electrons are found in an area known as the _____.
- neutronic membrane
- biosphere
- electron cloud
- nucleus
In an uncharged atom, the number of protons and _____ must be equal.
- electrons
- ions
- neurons
- particles
The _____ make(s) up about 99.9% of the mass of the atom.
- amu
- atomic number
- electron cloud
- nucleus
The 1st energy level of an atom can hold _____ electrons.
- 2
- 4
- 6
- 8
The 2nd energy level of an atom can hold _____ electrons.
- 4
- 6
- 8
- 12
The 3rd energy level of an atom can hold _____ electrons.
- 8
- 12
- 16
- 18
The 4th energy level of an atom can hold _____ electrons.
- 16
- 18
- 32
- 36
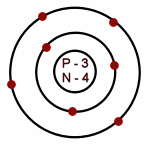
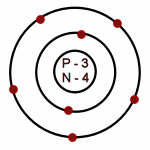
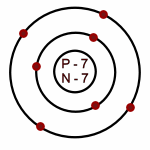
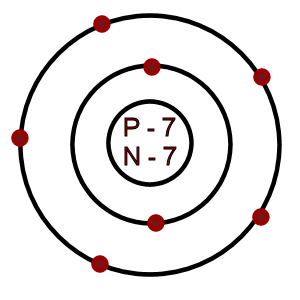
Nitrogen: Atomic # = 7
# of neutrons = 7
Which of the following is an accurate illustration of a nitrogen atom?
-
-
-
-
Neon: Atomic # = 10
# of neutrons = 10
Which of the following is an accurate illustration of a nitrogen atom?
-
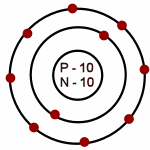
-
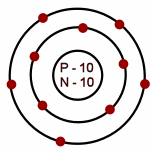
-
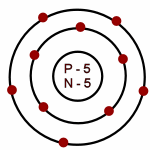
-